When you pick up a prescription, you might see two options: the name you recognize from TV ads, or a simpler label with a plain chemical name. One costs hundreds of dollars. The other, sometimes under a dollar. It’s easy to wonder-are generic drugs really the same? Or are you just saving money by risking your health?
The truth is simpler than the fear suggests. Generic drugs aren’t cheaper because they’re weaker. They’re cheaper because they don’t need to pay for ads, fancy packaging, or decades of research. But here’s what matters: they have to prove they work just as well. And they do.
What Makes a Generic Drug a Generic Drug?
A generic drug isn’t a copy. It’s a legal, FDA-approved twin. It has the same active ingredient, same strength, same form-tablet, capsule, injection-as the brand-name version. If your doctor prescribes lisinopril, the generic version of Zestril, it’s the exact same molecule doing the same job in your body.
The big difference? The inactive stuff. Fillers, dyes, coatings. These don’t affect how the drug works, but they can change how it looks or tastes. For most people, this doesn’t matter. But for a small number with allergies or sensitivities, those extras can cause reactions. That’s why pharmacists check your history before swapping.
The system that made this possible started in 1984 with the Hatch-Waxman Act. Before that, every new version of a drug had to go through the same expensive clinical trials as the original. That blocked competition. The law changed that. Now, generic makers only need to prove one thing: bioequivalence.
Bioequivalence: What It Really Means
Bioequivalence sounds technical, but it’s just a fancy way of saying: “Does this drug get into your blood the same way as the brand?”
To prove it, companies run tests with 24 to 36 healthy volunteers. They measure two things: how fast the drug reaches its highest level in your blood (Cmax), and how much of it gets absorbed over time (AUC). The generic’s numbers must fall within 80% to 125% of the brand’s. That’s it.
Let’s clear up a big myth: this doesn’t mean the generic has only 80% of the active ingredient. It means the way your body absorbs it can vary slightly-but still work just as well. In fact, studies show the average difference in absorption between generics and brands is just 3.5%. That’s less than the variation you get from eating a big meal before taking your pill.
The FDA rates generics with a letter system. Most are AB-rated, meaning they’re interchangeable with the brand. A few are B-rated, usually because they’re harder to make-like inhalers or creams. Those aren’t automatically interchangeable, but they’re still safe and effective. Your pharmacist will know which is which.
Cost Savings: The Numbers Don’t Lie
Brand-name drugs cost a lot because the company had to recover research, development, and marketing costs. Once the patent expires, anyone can make it. Competition kicks in. Prices drop.
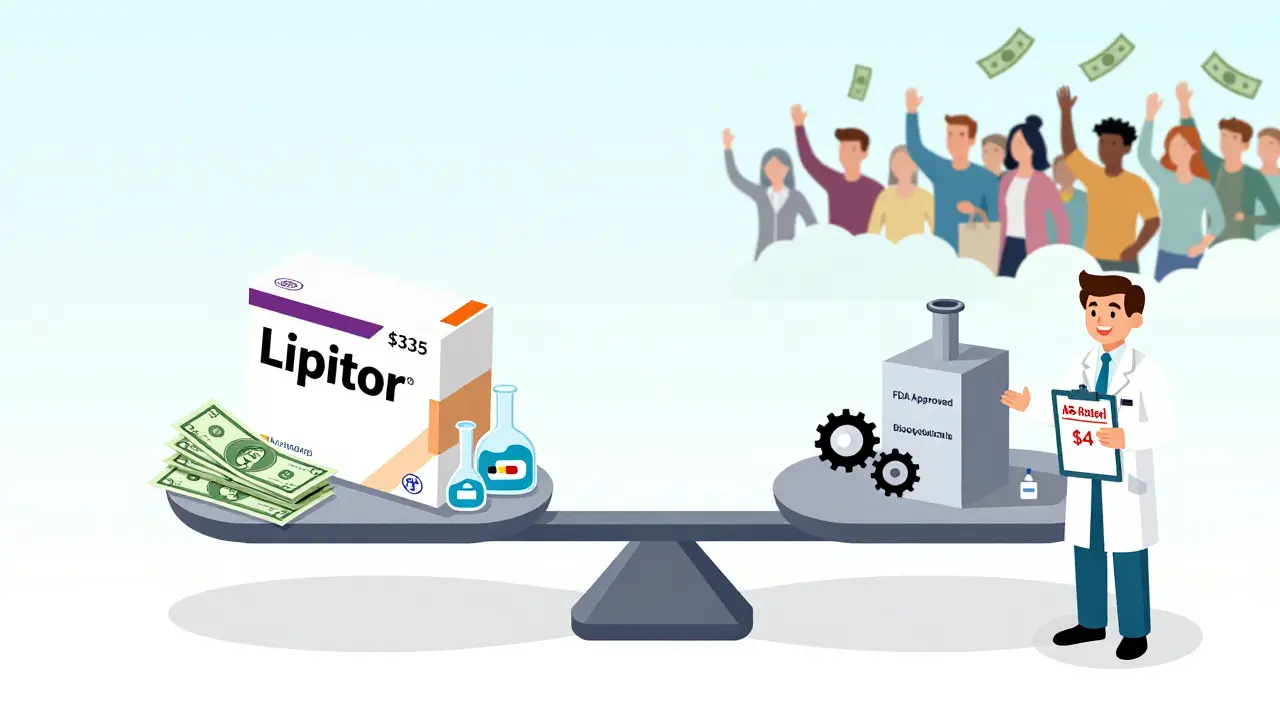
In 2023, generic drugs saved the U.S. healthcare system $373 billion. On average, they cost 80% to 85% less than the brand. Take Lipitor, the cholesterol drug. The brand version? Around $335 for a 30-day supply. The generic? Free with a coupon. Plavix, the blood thinner? $450 brand. $0 generic. These aren’t outliers. They’re the norm.
Even when you don’t have coupons, generics are still cheaper. A 30-day supply of metformin for diabetes? $4. Brand-name Glucophage? $120. That’s not a savings-it’s a life-changing difference for people on fixed incomes.
And it’s not just the U.S. Globally, generics made up $462 billion of the $1.12 trillion pharmaceutical market in 2023. They fill 90% of all prescriptions in America. But they only make up 23% of total drug spending. That’s the power of competition.
When Generics Might Need Extra Care
Most of the time, switching is safe. But there are exceptions. These are drugs with a narrow therapeutic index (NTI). That means even tiny changes in blood levels can cause big problems-either the drug stops working, or you get side effects.
Examples: warfarin (blood thinner), levothyroxine (thyroid hormone), phenytoin (seizure control), lithium (bipolar disorder), and digoxin (heart failure). For these, doctors and pharmacists often monitor blood levels closely after switching. It’s not because generics are unsafe-it’s because the margin for error is razor-thin.
A 2022 study found 61% of warfarin patients refused generic substitution, even though research showed no difference in outcomes. Fear, not science, drives that choice. The same study found only 32% of doctors knew levothyroxine was an NTI drug. That’s a gap in knowledge, not in safety.
The European Medicines Agency uses tighter standards for NTI drugs-90% to 111% instead of 80% to 125%. But even with looser U.S. rules, real-world data backs up safety. A massive 2020 study in Austria tracked 1.2 million patients on 17 different drugs. Generics performed just as well-or better-on death rates and heart events.
Why Do People Still Doubt Generics?
Despite the evidence, 43% of patients in a 2022 survey believed generics were less effective. One in four refused substitution when offered. Why?
Marketing. Brand-name companies spend billions on ads that make you think their version is “better.” They sponsor patient groups. They fund misleading studies. They make you feel like you’re taking a risk if you switch.
There’s also the placebo effect. If you believe the brand is stronger, you might feel worse when you switch-even if your blood levels haven’t changed. That’s real. But it’s psychological, not pharmacological.
On Reddit, a thread about generic vs. brand experiences got over 400 comments. Most people said they noticed no difference. But the ones who did? Often pointed to levothyroxine. That’s because thyroid hormone levels are sensitive. Even a 5% change in absorption can affect how you feel. But that doesn’t mean generics are bad. It means you need monitoring. Your doctor can adjust the dose if needed.
And here’s the kicker: GoodRx’s 2023 survey of over 8,000 users found 89% reported identical results with generics. The biggest reason people switched? Cost. The biggest reason they stayed? Fear.

What Should You Do?
If you’re on a brand-name drug and you’re worried about cost, ask your doctor or pharmacist: “Is there a generic?” If they say no, ask why. Is it because there isn’t one? Or because they’re used to prescribing the brand?
For most drugs-antibiotics, blood pressure pills, antidepressants, statins-switching is safe and smart. Use the FDA’s Orange Book to check if your drug has an AB-rated generic. Pharmacists use it daily to make substitutions.
If you’re on an NTI drug, don’t panic. Just ask for monitoring. A simple blood test two weeks after switching can confirm everything’s on track. Most insurance plans cover these tests.
And if you’ve had a bad experience with a generic? Tell your doctor. It might not be the drug-it might be the filler. Sometimes, switching to a different generic brand (yes, there are multiple) solves the issue.
Generics aren’t second-rate. They’re the result of smart policy, competition, and science. They’ve been used by millions for decades. They’re not perfect. But they’re safe, effective, and life-changing for people who can’t afford the brand.
The next time you see a prescription with two prices, remember: the cheaper one isn’t a compromise. It’s a victory-for your wallet, and for your health.
Are generic drugs as effective as brand-name drugs?
Yes. Generic drugs contain the same active ingredient, in the same strength and form, as the brand-name version. The FDA requires them to prove bioequivalence-meaning they deliver the same amount of medicine into your bloodstream at the same rate. Studies show no meaningful difference in effectiveness for the vast majority of drugs.
Why do generic drugs cost so much less?
Brand-name drug companies spend millions on research, clinical trials, and marketing to get approval and build awareness. Once the patent expires, other companies can make the same drug without repeating those costs. Generics only need to prove they work the same way, not re-prove safety. That’s why they’re 80-85% cheaper.
Can I switch from a brand-name drug to a generic safely?
For most medications, yes. The FDA approves generics for automatic substitution. But for drugs with a narrow therapeutic index-like warfarin, levothyroxine, or phenytoin-your doctor may want to monitor your blood levels after switching. This isn’t because generics are unsafe, but because small changes in blood levels can matter more with these drugs.
Do generics have the same side effects as brand-name drugs?
Yes. Since they contain the same active ingredient, the risks and side effects are identical. The only difference might come from inactive ingredients-like dyes or fillers-which can rarely cause allergic reactions. If you notice a new side effect after switching, tell your doctor. It might be the filler, not the medicine.
How do I know if my generic drug is FDA-approved and safe?
All FDA-approved generics are safe. Look for the AB rating in the FDA’s Orange Book, which lists therapeutically equivalent drugs. Your pharmacist can tell you if your generic is AB-rated. If it’s not, they’ll explain why. You can also check the FDA’s website or ask your doctor to confirm approval status.
Why do some people say generics don’t work for them?
Sometimes, it’s psychological-people expect a difference and feel one. Other times, it’s because they switched between two different generic brands, and the inactive ingredients changed. Rarely, a specific generic might have a formulation issue, but that’s uncommon. If you feel worse after switching, talk to your doctor. They can check your blood levels or try a different generic version.
Are there any drugs that don’t have generic versions?
Yes, but not many. New drugs are protected by patents for 20 years. Some complex drugs-like biologics, inhalers, or topical creams-take longer to develop generics for. But over 90% of prescriptions in the U.S. are for drugs with generic alternatives. If your drug doesn’t have one yet, it likely will in the next few years.
Can I ask my pharmacist to give me the brand-name drug instead?
Yes, but you’ll pay more. Pharmacists are required to substitute with a generic unless the prescription says “Dispense as Written” or “Do Not Substitute.” If you want the brand, you can request it-but you’ll likely pay the full price, not the generic copay. Some insurance plans won’t cover the brand unless you prove the generic doesn’t work for you.
What’s Next for Generic Drugs?
The FDA is pushing harder to approve complex generics-like inhalers and injectables-that are harder to copy. In 2023, they approved 247 of these, up 19% from the year before. New technology is making manufacturing more precise. A 2023 MIT study showed future generics for warfarin could reduce absorption variation to under 2%.
But there’s a problem: shortages. In 2023, there were 312 generic drug shortages in the U.S., mostly sterile injectables. That’s up 17% from 2022. Many come from overseas factories. Supply chain issues can leave patients without access-even if the drug is technically available.
For now, generics remain the best tool we have to make medicine affordable. They’re not perfect. But they’re the closest thing to a win-win in healthcare: same effectiveness, lower cost, and millions of people using them every day without issue.





Beth Beltway
January 29, 2026 AT 20:38Marc Bains
January 30, 2026 AT 09:39calanha nevin
January 30, 2026 AT 13:15Mike Rose
January 31, 2026 AT 17:36Russ Kelemen
January 31, 2026 AT 21:27Diksha Srivastava
February 2, 2026 AT 10:06Sidhanth SY
February 3, 2026 AT 08:27Adarsh Uttral
February 3, 2026 AT 20:21Niamh Trihy
February 5, 2026 AT 01:12Jason Xin
February 6, 2026 AT 05:20Donna Fleetwood
February 6, 2026 AT 22:59Bobbi Van Riet
February 8, 2026 AT 12:45Shubham Dixit
February 9, 2026 AT 09:54KATHRYN JOHNSON
February 10, 2026 AT 01:01Sazzy De
February 10, 2026 AT 20:15