TRIPS Agreement: How Global Patent Rules Shape Drug Access and Prices
When you hear TRIPS Agreement, the international treaty that sets minimum standards for intellectual property rights including pharmaceutical patents. Also known as Agreement on Trade-Related Aspects of Intellectual Property Rights, it’s the rulebook that decides who can copy life-saving medicines and when. This isn’t just legal jargon—it’s the reason some drugs cost thousands in the U.S. but pennies in India or Kenya.
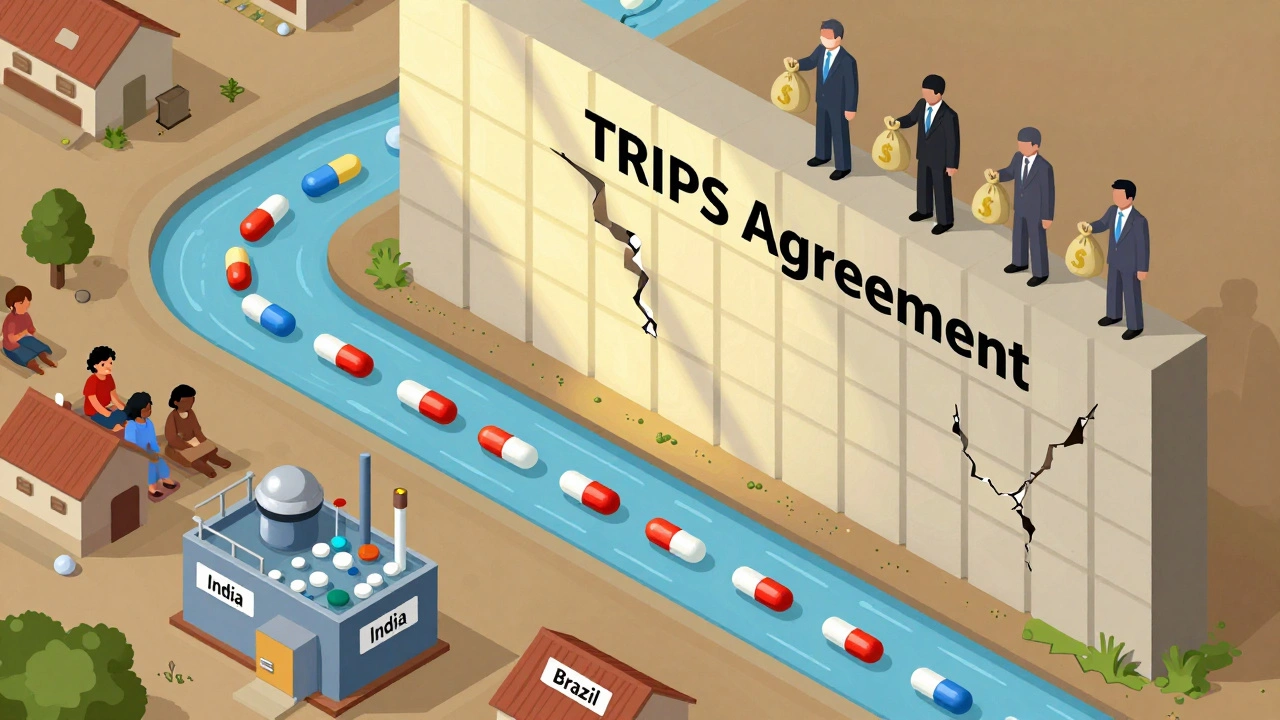
The TRIPS Agreement, a WTO-mandated framework adopted in 1995 forces countries to grant 20-year patents on new drugs. That means no one else can make a copy until the patent expires—even if millions can’t afford the brand-name version. But here’s the twist: the agreement lets developing nations issue compulsory licenses to produce or import cheaper generics during public health emergencies. That’s how countries like South Africa and Brazil got access to affordable HIV drugs in the early 2000s, despite pushback from big pharma.
It also connects directly to how generic drugs, medications that contain the same active ingredients as brand-name drugs but cost far less enter the market. In the U.S., the Hatch-Waxman Act, a law that balances patent protection with generic competition lets companies file for approval before a brand’s patent ends, but only after legal battles in the Federal Circuit Court, the court that handles all pharmaceutical patent disputes in the U.S.. These legal fights delay generics for years—sometimes by claiming new dosing methods or formulations that aren’t truly innovative.
And it’s not just about cost. The TRIPS Agreement, the global standard for drug patent rules has shaped how African nations now produce their own antiretrovirals. By using flexibilities built into the treaty, countries like Nigeria and Ethiopia are manufacturing HIV meds locally—cutting dependence on imports and lowering prices. That’s not charity. It’s the TRIPS Agreement working as intended: protecting innovation while allowing access when lives are on the line.
But here’s the problem: many countries still don’t know how to use these flexibilities. Or they’re pressured not to. The result? A system where patent lawyers often decide who gets treated—not doctors or patients. That’s why understanding the TRIPS Agreement matters. It’s the invisible hand behind your prescription price tag, your access to generics, and whether a child in a low-income country can get the same medicine as one in New York.
Below, you’ll find real-world examples of how this agreement plays out—from patent battles that delay affordable drugs, to how countries fight back, to why some generics still struggle to gain trust even when they’re legally approved. These aren’t abstract policies. They’re the reason your insulin costs $300—or $3.
The TRIPS Agreement enforces global pharmaceutical patents that block affordable generic drugs, leaving millions without life-saving treatments. Despite legal flexibilities, complex rules and political pressure make access nearly impossible for low-income countries.
View More