Patent Rights in Pharmaceuticals: How They Shape Drug Prices and Access
When you hear patent rights, legal protections that give drug companies exclusive rights to sell a medicine for a set time. Also known as drug exclusivity, these rights are the foundation of how pharmaceutical companies recoup billions in research costs—and how they keep prices high for years. Without patent rights, any company could copy a new drug the day it’s approved. But with them, only the original maker can sell it, often at prices ten times higher than what it costs to produce.
These rights don’t last forever. In the U.S., most drug patents run for 20 years from the date they’re filed—but that’s not the whole story. Many companies file multiple patents on the same drug: one for the active ingredient, another for the pill shape, another for how it’s taken, even for the packaging. This practice, called evergreening, extending market exclusivity by filing new patents on minor changes, can delay generic versions by decades. The Hatch-Waxman Act, a 1984 law that balances innovation with access by letting generics enter after patent expiry was designed to fix this. It created a fast track for generic manufacturers to prove their versions work the same, without repeating expensive clinical trials. But loopholes still exist. Some companies sue generic makers over patents that courts later throw out, buying more time to keep prices up.
When a patent expires, the market shifts fast. Generic versions flood in, often cutting prices by 80% or more—like what happened with simvastatin or metformin. But not all drugs follow the same path. Some get extended protection through special rules, like orphan drug status for rare diseases, or data exclusivity that blocks generics even after the patent runs out. And in places like Africa, where local manufacturers produce antiretroviral generics, patent rights are being challenged to save lives. The Federal Circuit Court, the only U.S. court that handles pharmaceutical patent cases decides who wins these battles—and its rulings directly affect whether you can afford your meds next year.
What you’ll find here are real stories behind those patent battles: how dosing claims get rejected, why some generics take years to appear, how grapefruit juice can trigger legal headaches, and why your doctor might not know if your pill is brand or generic because the label doesn’t say. These aren’t abstract legal concepts. They’re the reason some drugs cost hundreds of dollars while others cost a few dollars. And they’re the reason you might be paying more than you need to—unless you know how to ask the right questions.
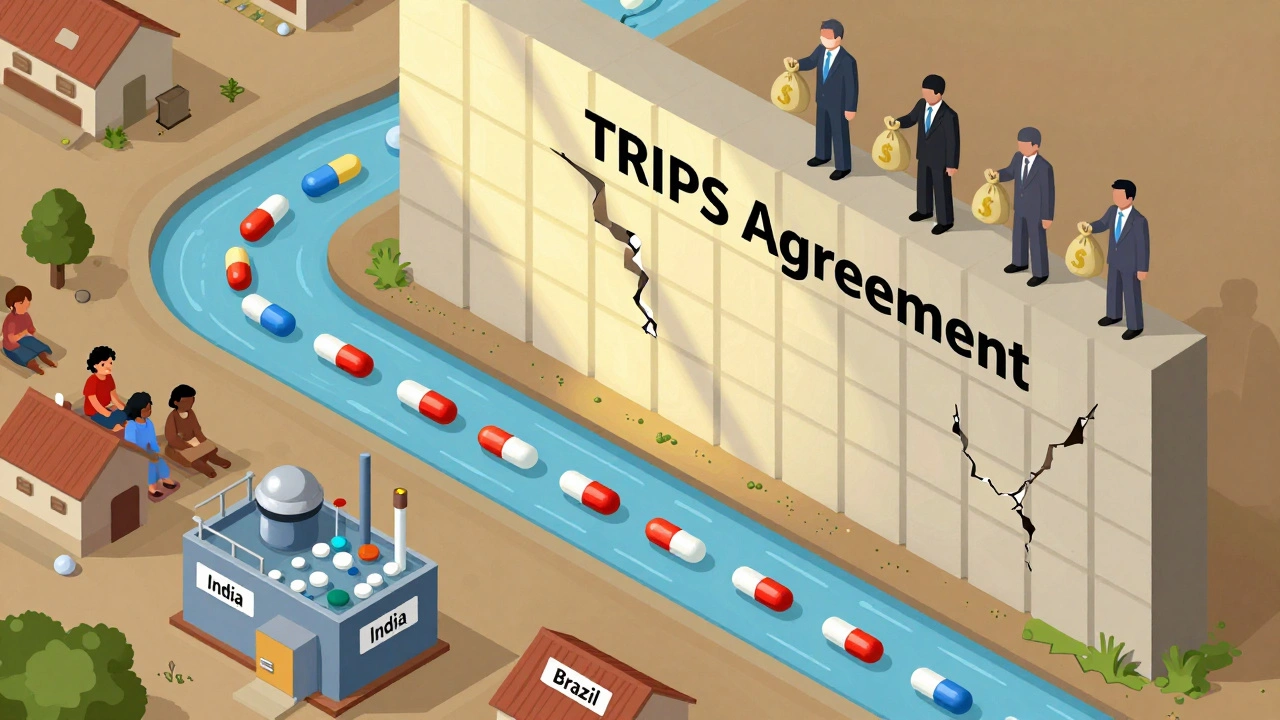
The TRIPS Agreement enforces global pharmaceutical patents that block affordable generic drugs, leaving millions without life-saving treatments. Despite legal flexibilities, complex rules and political pressure make access nearly impossible for low-income countries.
View More