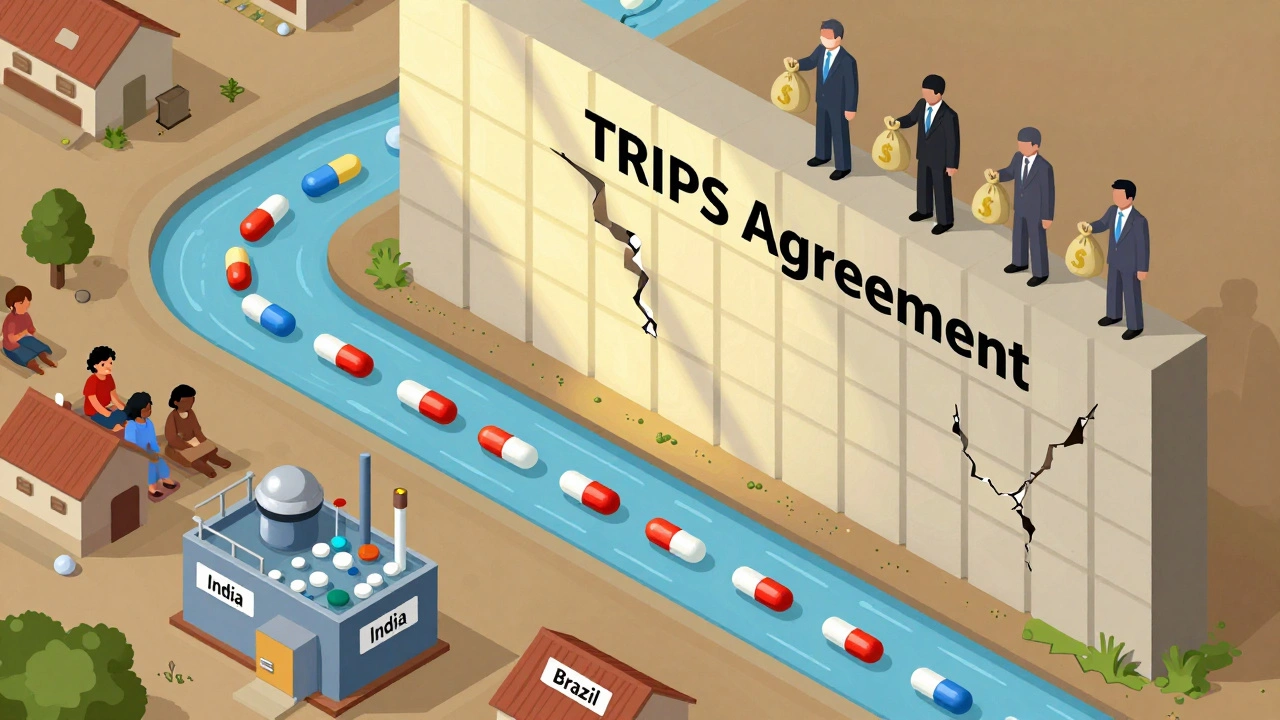
Compulsory Licensing: How Governments Override Patents to Lower Drug Prices
When a life-saving drug is priced out of reach, compulsory licensing, a legal tool that lets governments authorize generic production without the patent holder’s consent. Also known as government use licenses, it’s not about stealing intellectual property—it’s about saving lives when the system fails. This isn’t theoretical. Countries like India, Brazil, and South Africa have used it to make HIV drugs, cancer treatments, and hepatitis C medicines affordable for millions. The World Health Organization supports it as a legitimate part of global health policy under the TRIPS Agreement.
Compulsory licensing doesn’t mean ignoring patents entirely. It means balancing them with public need. When a drug costs $100,000 a year and a country can’t afford it, the law allows local manufacturers to produce a generic version at a fraction of the price—sometimes under 10% of the original. The patent holder still gets paid, but at a rate set by the government, not the company. This is how countries like Thailand cut the price of the HIV drug efavirenz from $1,000 to $50 per patient per year. It’s also how Rwanda and other African nations access affordable antiretrovirals made locally, thanks to partnerships with generic manufacturers and WHO-prequalified supply chains.
It’s not just about low-income countries. Even wealthy nations like Canada and Germany have used compulsory licensing for high-cost drugs, especially when manufacturers refuse to negotiate fair prices. The U.S. has never used it broadly for pharmaceuticals, but it’s been discussed during the COVID-19 pandemic and in debates over insulin pricing. Meanwhile, the Federal Circuit Court, the U.S. court that handles all pharmaceutical patent disputes often hears cases where companies fight generic makers over patent loopholes—sometimes trying to block licensing before it even starts. That’s why understanding compulsory licensing means also understanding the legal battleground where drug prices are decided.
Compulsory licensing connects directly to how generic drugs, medications that contain the same active ingredients as brand-name drugs but cost far less enter the market. Most generics arrive through legal challenges or patent expirations, but when time is critical—like during a pandemic or a public health emergency—compulsory licensing is the fast track. It bypasses years of litigation and lets production begin immediately. That’s why it’s not just a legal tool—it’s a public health necessity.
What you’ll find in the posts below are real-world examples of how drug pricing, patent law, and access intersect. From how African countries produce their own HIV meds to how U.S. courts shape patent battles, these stories show that affordable medicine isn’t just about cost—it’s about power, policy, and who gets to decide what’s fair.
The TRIPS Agreement enforces global pharmaceutical patents that block affordable generic drugs, leaving millions without life-saving treatments. Despite legal flexibilities, complex rules and political pressure make access nearly impossible for low-income countries.
View More