Switching from brand-name Dilantin to a generic version of phenytoin might seem like a simple cost-saving move. But for patients taking this drug, it’s not just a substitution-it’s a potential safety issue. Phenytoin isn’t like most medications. Even small changes in blood levels can trigger seizures or cause serious toxicity. And because of how it’s absorbed and processed in the body, generic versions-even those approved by the FDA-can behave differently enough to put patients at risk.
Why phenytoin is different
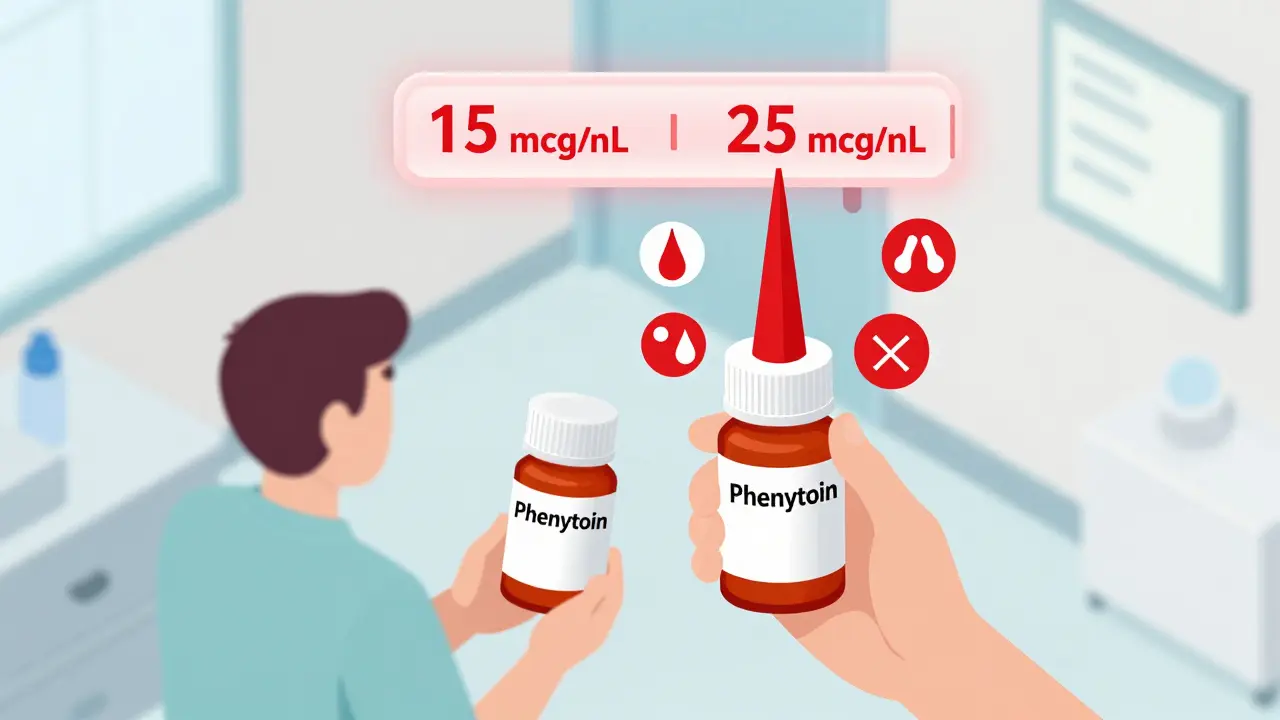
Phenytoin has a narrow therapeutic index. That means the difference between a dose that works and one that’s dangerous is tiny. The safe, effective range is 10 to 20 mcg/mL. Go below 10, and seizures might return. Go above 20, and you risk confusion, uncontrolled eye movements, loss of coordination, and even coma or death. At levels over 50 mcg/mL, fatal toxicity becomes likely. What makes phenytoin extra tricky is its non-linear pharmacokinetics. Normally, if you double the dose, the blood level doubles. With phenytoin, that doesn’t happen. Once the body’s enzymes get saturated-usually around 10-20 mcg/mL-small increases in dose cause big jumps in concentration. A 25-50 mg increase might push levels from 18 to 35 mcg/mL overnight. That’s not a typo. That’s a medical emergency. Add to that: phenytoin is 90-95% bound to proteins in the blood. Only the 5-10% that’s free is active. So if a patient has low albumin-common in older adults, liver disease, or malnutrition-the total phenytoin level might look normal, but the free, active part could be dangerously high. That’s why total levels can be misleading.Generic substitutions aren’t always equal
The FDA allows generic drugs to differ from the brand by up to 20% in how much of the drug gets into the bloodstream (AUC) and how fast it gets there (Cmax). For most drugs, that’s fine. For phenytoin? Not even close. Imagine two generic versions: one releases phenytoin slowly, another quickly. Both meet FDA standards. But one might push levels from 15 to 22 mcg/mL after a switch. The other keeps it steady at 16. The first one could cause toxicity. The second might be safe. Yet both are labeled "bioequivalent." This isn’t theoretical. Clinicians in Australia, the UK, and the U.S. have reported cases where patients had breakthrough seizures or new side effects after switching between generic brands. Some patients tolerate one generic just fine but react badly to another-even if both come from the same manufacturer but different batches. The NHS Tayside guideline says it plainly: "Therapeutic monitoring may be required when switching formulations." The American Academy of Family Physicians agrees: while routine monitoring isn’t needed for everyone, it’s essential when changing phenytoin products.When and how to monitor
Don’t wait for symptoms to appear. Monitor before, during, and after any switch.- Before switching: Get a trough level (just before the next dose). This is your baseline.
- After switching: Wait at least 5 days before checking again. Phenytoin takes time to reach steady state. A level taken too early (within 3-4 days) won’t reflect true exposure.
- After a dose change: Check again at day 5-10. If the level is outside 10-20 mcg/mL, adjust the dose slowly-no more than 25-50 mg at a time.
- For IV loading: If given intravenously, check levels 2-4 hours after the dose. For oral, wait 12-24 hours.

Other factors that change phenytoin levels
It’s not just generics. Many common drugs mess with phenytoin.- Drugs that raise phenytoin: Fluconazole, metronidazole, cimetidine, amiodarone, valproate, and even some antibiotics like sulfamethoxazole.
- Drugs that lower phenytoin: Rifampin, carbamazepine, alcohol, and theophylline.
Long-term monitoring beyond blood levels
Phenytoin doesn’t just affect your brain. It affects your bones, gums, and blood.- Gingival hyperplasia: Swollen, overgrown gums are common. Good dental hygiene helps, but it’s not always preventable.
- Bone health: Phenytoin speeds up vitamin D breakdown. Over time, this leads to low calcium, low phosphate, and weak bones (osteomalacia). Check vitamin D, calcium, and alkaline phosphatase every 2-5 years.
- Blood counts: Phenytoin can suppress bone marrow. Monitor full blood count regularly, especially in the first few months.
- Genetic risk: For patients of Han Chinese or Thai descent, test for the HLA-B*1502 allele before starting. This gene variant increases the risk of a deadly skin reaction called SJS/TEN.
What to do if you’re switching
If you’re a patient or caregiver:- Ask your pharmacist: "Is this the same brand as before?" If it’s different, ask if you can stay on the original.
- Don’t assume the new pill works the same. Even if the label says "phenytoin sodium 100 mg," the formulation might be different.
- Watch for new side effects: dizziness, slurred speech, unsteady walking, nausea, or confusion.
- Call your doctor if you have a seizure after switching-even if it’s just one.
- Request a blood level check 5-10 days after the switch.
- Document the exact manufacturer and batch number of the phenytoin being prescribed.
- Never switch without a baseline level.
- Use free phenytoin testing in patients with low albumin, liver disease, or critical illness.
- Warn patients: "This isn’t like switching painkillers. Even a small change can be dangerous."
Bottom line
Generic phenytoin is cheaper. But it’s not interchangeable in the way most generics are. For this drug, bioequivalence doesn’t mean clinical equivalence. The narrow window, nonlinear kinetics, and high protein binding make phenytoin uniquely sensitive to formulation changes. Therapeutic drug monitoring isn’t optional here-it’s essential. Not for every patient, every time. But when you switch products? Always. When levels are near the edge? Always. When the patient is elderly, sick, or on other meds? Always. Don’t let cost savings become a patient safety risk. Check the level. Watch the symptoms. Know the drugs that interact. Monitor the bones and gums. Phenytoin is old, but it’s still powerful. And it demands respect.Can I switch between generic phenytoin brands without checking blood levels?
No. Even though both products are labeled "phenytoin," different manufacturers use different fillers and coatings that affect how the drug is absorbed. For a drug with phenytoin’s narrow therapeutic index and non-linear metabolism, even small changes in absorption can push levels into the toxic range. Always check a trough level 5-10 days after switching, regardless of whether it’s brand to generic or generic to generic.
Why is free phenytoin testing better than total phenytoin in some patients?
Phenytoin is 90-95% bound to proteins in the blood. Only the unbound (free) portion crosses into the brain to work. In patients with low albumin-due to liver disease, malnutrition, or kidney failure-the total phenytoin level may look normal, but the free fraction can be dangerously high. Free phenytoin testing measures exactly what’s active in the body, avoiding false reassurance from total levels.
How long does it take for phenytoin to reach steady state after a dose change?
It takes about 7-10 days for phenytoin to reach steady state in most adults because of its long half-life (around 24 hours) and saturation kinetics. Levels checked before day 5 are unreliable. Always wait at least 5 days after a dose change or formulation switch before drawing a level for interpretation.
What are the signs of phenytoin toxicity?
Early signs include nystagmus (involuntary eye movements), dizziness, slurred speech, and loss of coordination. As levels rise above 30 mcg/mL, patients may become confused or drowsy. Above 40 mcg/mL, mental status declines significantly. Above 50 mcg/mL, seizures and death can occur. If any of these symptoms appear after a dose change or switch, check the level immediately.
Do I need to monitor vitamin D and bone health if I’m on phenytoin?
Yes. Phenytoin increases the breakdown of vitamin D, which can lead to low calcium, low phosphate, and weak bones (osteomalacia). This happens over years, not weeks. Check vitamin D, calcium, and alkaline phosphatase every 2-5 years. Consider supplements if levels are low, especially in older adults or those with limited sun exposure.
Can I stop phenytoin if I’m worried about side effects?
Never stop phenytoin suddenly. Abrupt withdrawal can trigger status epilepticus, a life-threatening seizure emergency. If side effects are a concern, talk to your doctor. They can adjust the dose slowly, switch to a different antiepileptic, or monitor levels more closely. Never make changes on your own.

Naomi Lopez
December 17, 2025 AT 17:46Let’s be real-this isn’t just about generics. It’s about how we’ve normalized cutting corners in healthcare while pretending everything’s fine. Phenytoin isn’t some generic aspirin. It’s a precision instrument, and we’re treating it like a Walmart flashlight. The FDA’s 20% bioequivalence buffer? Absurd. For a drug with nonlinear kinetics and 95% protein binding, that’s like saying two different rockets can land on Mars if they’re both ‘close enough.’
And don’t get me started on the myth that ‘it’s just phenytoin.’ No. It’s the excipients. The coating. The dissolution profile. One batch’s filler could be microcrystalline cellulose; another’s might be lactose monohydrate with a different particle size. Tiny differences. Catastrophic consequences.
I’ve seen patients crash into toxicity after a pharmacy substitution. No warning. No notice. Just a new pill bottle and a seizure three days later. We need mandatory batch tracking. We need prescribers to specify the manufacturer. We need to stop pretending this is a cost-saving measure and not a gamble with lives.
And yes, I’m aware this will never change. But that doesn’t make it right.
Salome Perez
December 18, 2025 AT 22:29Thank you for this meticulously crafted guide-it reads like a love letter to clinical wisdom. Phenytoin, in all its finicky glory, deserves reverence. I’ve worked in neurology for over two decades, and I still shudder at the thought of a pharmacist swapping out a patient’s phenytoin without a whisper of warning.
Free phenytoin testing is not a luxury-it’s a lifeline, especially for our elderly patients with hypoalbuminemia. I’ve had cases where total levels were ‘perfect’ at 14 mcg/mL… yet the free fraction was 3.8 mcg/mL (toxic). The patient was slurring words, eyes darting like a startled bird. We switched back immediately.
And yes, HLA-B*1502 screening? Mandatory. In my clinic, we screen everyone of Southeast Asian descent before the first prescription. It’s not optional. It’s ethical.
Also-gingival hyperplasia. So many patients think it’s just ‘bad brushing.’ It’s not. It’s pharmacological. We should be handing out dental floss with every script.
Thank you for reminding us that some drugs are not interchangeable. Some are sacred.
Erik J
December 20, 2025 AT 08:06Interesting. I’m curious-how often do hospitals actually do free phenytoin tests? I’ve seen the lab orders, but I’ve never seen one actually processed. Is it because of cost? Or just inertia?
Also, the formula for corrected phenytoin-does it hold up in real-world scenarios, or is it just a rough heuristic? I’ve seen it used in ERs, but I wonder if it’s more misleading than helpful in acute settings.
Martin Spedding
December 20, 2025 AT 19:14Bro this is why america’s healthcare is trash. You pay 300 bucks for dilantin, then they switch you to some generic made in india for $12 and you get seizures? LMAO. WHO CARES ABOUT THE FORMULATION?? JUST TAKE THE PILLS.
Also free phenytoin test? That’s like 200 bucks?? WASTED. Just give them more dilantin and shut up.
Also why are you even talking about albumin? Just give them protein shakes. Problem solved. 😎
Sam Clark
December 21, 2025 AT 14:16This is one of the most clinically vital summaries I’ve read in years. Thank you for the precision and clarity.
As a pharmacist, I’ve seen firsthand how easily a switch can go unnoticed-especially in long-term care facilities where med passes are rushed and documentation is sparse. I now insist on a signed form from the prescriber when phenytoin is dispensed, specifying manufacturer and batch number. It’s extra work, but it’s non-negotiable.
And I echo the point about HLA-B*1502. We’ve started screening all new patients with South Asian ancestry. One positive result prevented a potential SJS/TEN case. That one test saved a life.
Let’s not normalize this risk. Phenytoin deserves better.
Jessica Salgado
December 22, 2025 AT 20:55I’m a caregiver for my brother who’s been on phenytoin since he was 12. He had a seizure last year after a pharmacy switch. We didn’t even know they changed it until we saw the new bottle. He was in the hospital for three days. I screamed at the pharmacist. No one apologized.
Now I call the pharmacy every single time he gets a refill. I ask: ‘Is this the same as last time?’ If they say ‘yes’ but the pill looks different? I refuse it. I don’t care if it’s cheaper. I don’t care if it’s ‘FDA approved.’
And yes-I demand free phenytoin levels now. I’ve learned the hard way that ‘normal’ doesn’t mean safe.
Why is this not common knowledge? Why are we still letting this happen? 🥺
Virginia Seitz
December 23, 2025 AT 17:28OMG YES. I just had this happen to my mom 😭 She switched generics and started stumbling around like a drunk penguin. We thought she had a stroke. Turned out her phenytoin level was 38. Now she’s back on the original brand. I’m never letting them switch again. 🙏💊 #PhenytoinIsNotAspirin
Sachin Bhorde
December 25, 2025 AT 10:43Bro, phenytoin is a beast. Nonlinear kinetics? Protein binding? Yeah, man. I’ve seen this in my neuro unit in Mumbai. One batch from a local pharma-patients started with ataxia. Next batch? Fine. We had to track batch numbers manually because the system didn’t. Free levels? We don’t have them here. So we rely on clinical signs. Nystagmus = stop. Dizziness = stop. Slurred speech = STOP.
Also, HLA-B*1502? We screen everyone. It’s not expensive. It’s life-saving. Why aren’t we doing this everywhere?
And yes-gingival hyperplasia. We give them toothbrushes. That’s it. No one teaches them. Sad.
Kent Peterson
December 25, 2025 AT 14:03THIS IS WHY WE NEED TO STOP LETTING FOREIGN COMPANIES MAKE OUR MEDS. WHO LETS A GENERIC FROM CHINA BE ‘BIOEQUIVALENT’ TO DILANTIN??
IT’S NOT A ‘FORMULATION DIFFERENCE’-IT’S A PLOT. THE PHARMA INDUSTRY IS IN BED WITH THE FDA. THEY WANT YOU TO DIE SO THEY CAN SAVE 50 CENTS.
WE NEED TO BAN ALL GENERIC PHENYTOIN. MAKE THEM ALL USE DILANTIN. OR WE’LL SEE A REVOLUTION.
AND WHY IS THIS POST SO LONG?? I’M SICK OF THIS ‘CLINICAL WISDOM’ BULLSHIT. JUST TELL ME WHAT TO DO.
STOP OVERCOMPLICATING. JUST GIVE ME THE BRAND.
AMERICA IS WEAK.
Josh Potter
December 27, 2025 AT 06:44Bro I just got switched to generic phenytoin last week. Thought I was saving cash. Now I’m dizzy 24/7 and my eyes are doing the cha-cha. Called my doc. They said ‘oh yeah, that happens.’
So I asked for my old brand. They said ‘it’s not covered.’
I’m filing a complaint. This isn’t healthcare. It’s a casino.
Also-free phenytoin test? I’m getting one tomorrow. If they charge me, I’m suing. I’m not dying because of a pill’s filler.
PS: I’m sharing this everywhere. #PhenytoinEmergency
Evelyn Vélez Mejía
December 28, 2025 AT 17:55Phenytoin is not merely a drug. It is a mirror held up to the moral architecture of modern medicine. We speak of bioequivalence as if it were a scientific certainty, when in truth it is a statistical fiction-engineered to satisfy regulatory convenience, not biological truth.
When we allow a drug with nonlinear kinetics and protein saturation to be treated as interchangeable, we are not practicing medicine. We are commodifying human physiology.
The patient’s brain does not care about FDA guidelines. It cares about free drug concentration. The liver does not care about cost-saving metrics. It cares about enzyme saturation.
This is not about generics. This is about whether we still believe that human life deserves precision-or merely efficiency.
The answer, I fear, is written in the seizures we ignore.
Victoria Rogers
December 30, 2025 AT 15:55Ugh. Another ‘Phenytoin is special’ post. Newsflash: EVERY drug has quirks. Why is this one getting the royal treatment?
Also, free phenytoin? That’s a scam. Labs make money off it. They’re pushing it to boost revenue.
And HLA-B*1502? That’s just for Asians. Why aren’t we testing everyone? Hypocrites.
Also, why is this even a thing? Just use levetiracetam. It’s cheaper, safer, and doesn’t need 17 blood tests a year. This whole post feels like fearmongering to sell more lab work.
Also-why are you even talking about albumin? Just give them more protein. Done.
Stop overcomplicating. Stop scaring people. Phenytoin is fine. You’re just scared of change.