For people with autoimmune diseases like rheumatoid arthritis, psoriasis, or alopecia areata, the shift from monthly injections to a daily pill has been life-changing. JAK inhibitors-oral drugs that quietly block inflammation at the cellular level-are now a major part of treatment plans. But they’re not simple pills. They come with serious risks, strict monitoring rules, and a lot of unanswered questions about long-term safety. If you’re considering one-or already taking it-here’s what you actually need to know.
How JAK Inhibitors Work (Without the Jargon)
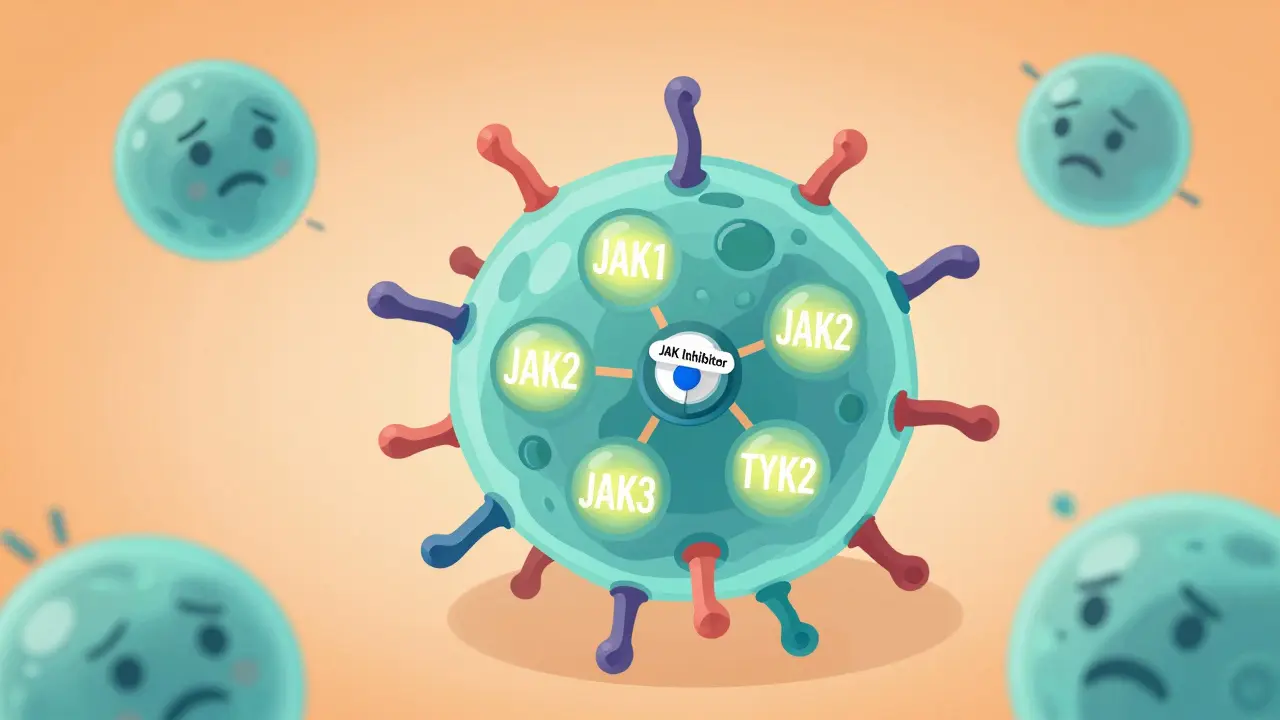
Your immune system uses tiny signals called cytokines to tell cells when to attack invaders. But in autoimmune diseases, those signals get stuck on. JAK inhibitors stop those signals before they even start. They target enzymes inside cells called Janus kinases (JAKs), which act like switches in the inflammation pathway. When a cytokine binds to a cell, it turns on a JAK enzyme, which then flips on other proteins that trigger immune responses. JAK inhibitors plug into the enzyme’s active site, like jamming a key in a lock, and shut down the whole chain.
There are four JAK enzymes: JAK1, JAK2, JAK3, and TYK2. Different drugs block different combinations. For example, upadacitinib is highly selective for JAK1, which helps reduce inflammation without messing up blood cell production as much. Abrocitinib targets JAK1 and JAK2, making it powerful for skin conditions like atopic dermatitis. Ritlecitinib is unique-it binds permanently to JAK3, which is why it works so well for alopecia areata. This precision matters. The more selective the drug, the fewer side effects you’re likely to get.
Why They’re a Big Deal Compared to Biologics
Before JAK inhibitors, most targeted treatments were biologics-injectable proteins made from living cells. These work well, but they’re expensive, require shots or infusions, and can take months to show results. JAK inhibitors are different. They’re small molecules you swallow. Most people feel better in 2 to 4 weeks, not 8 to 12. In clinical trials, upadacitinib reduced joint swelling in 71% of rheumatoid arthritis patients at 12 weeks. That’s nearly double the placebo rate.
They also work across multiple conditions. A patient with both rheumatoid arthritis and psoriasis can often treat both with one pill. That’s huge. In 2023, JAK inhibitors captured about 25% of the rheumatoid arthritis market in the U.S., and that number is still climbing. For many, the convenience outweighs the risks-especially when biologics have failed.
The Dark Side: Black Box Warnings and Real Risks
But here’s the catch. In January 2022, the FDA added a black box warning-the strongest possible-to all JAK inhibitors. That means: serious infections, cancer, heart attacks, strokes, and blood clots are real dangers. The ORAL Surveillance study, which tracked over 4,000 rheumatoid arthritis patients over 8 years, found tofacitinib increased the risk of major cardiovascular events by 31% and cancer by 49% compared to TNF inhibitors, especially in patients over 50 with existing heart disease or a smoking history.
One of the most common side effects? Herpes zoster-shingles. About 23% of users on JAK inhibitors report it, compared to just 3% on biologics. Many end up on daily antiviral pills just to prevent outbreaks. Others notice their cholesterol spikes. One Reddit user reported his LDL jumped 28 mg/dL after starting abrocitinib. That’s enough to push him into the high-risk category, requiring a statin.
And it’s not just numbers. People are scared. A 68-year-old woman on baricitinib told her rheumatologist she’d rather live with stiff joints than risk a stroke. Another man, after two shingles episodes in six months, stopped his JAK inhibitor cold. He’s now on a biologic again. These aren’t rare stories. They’re common enough that doctors now spend half their appointments explaining risk versus benefit.
Who Should and Shouldn’t Take Them
Not everyone is a candidate. The European League Against Rheumatism and the American College of Rheumatology now agree: avoid JAK inhibitors if you’re over 65, have a history of heart disease, stroke, blood clots, or cancer. Smokers? High cholesterol? Uncontrolled diabetes? Those are red flags too. In Europe, only 18% of rheumatologists prescribe them as first-line after methotrexate. In the U.S., it’s 32%. Why the difference? Europe is stricter. They wait until biologics fail first. The U.S. moves faster.
They’re also not for people with low white blood cell counts, liver problems, or active infections. Before starting, you need a full blood panel, TB test, hepatitis screening, and a lipid check. If your lymphocyte count is below 500 cells/μL, you shouldn’t start. If your ALT or AST levels are more than three times the normal range, you need to pause or switch. These aren’t suggestions-they’re requirements.
Monitoring: What You Need to Track and When
Monitoring isn’t optional. It’s mandatory. The first year, you need blood tests every three months. After that, every six months. Here’s what’s checked:
- Complete blood count: Watch for low white cells, red cells, or platelets. If your lymphocyte count drops below 500, stop the drug.
- Liver enzymes (ALT/AST): If they rise above 3x the upper limit, you need to pause treatment.
- Lipid panel: LDL over 190 mg/dL? Start a statin. Many patients need this within 3 months.
- Hemoglobin: If it falls below 8 g/dL, that’s a sign of anemia and requires action.
- Shingles screening: If you’ve never had chickenpox or the vaccine, get vaccinated-at least 4 weeks before starting. But here’s the problem: 68% of clinics in Europe skip this because they’re rushing to treat patients.
Some doctors now use digital tools to flag abnormal results automatically. Others still rely on paper charts and patient memory. Either way, if you miss a blood test, you’re playing Russian roulette with your health.
What’s New in 2024 and What’s Coming
The latest FDA approval was deuruxolitinib for alopecia areata in June 2024. It’s the first JAK inhibitor approved specifically for hair loss-and it comes with a mandatory REMS program. That means you can’t get it without enrolling in a safety tracking system. You’ll need regular blood tests and signed consent forms.
Next up? Highly selective TYK2 inhibitors like brepocitinib, expected to complete phase 3 trials in mid-2025. These promise to block inflammation without affecting JAK2, which controls red blood cell and platelet production. That could mean fewer anemia and clotting issues. There’s also ritlecitinib, which binds permanently to JAK3. It’s already used for alopecia, but trials are expanding to other conditions.
And while JAK inhibitors are being used off-label for vitiligo and hidradenitis suppurativa, the data is still early. Dermatologists report good results, but long-term safety? Unknown.
The Bottom Line: Power With Responsibility
JAK inhibitors are powerful. They’ve given people their lives back-clear skin, no more swollen joints, regrown hair. But they’re not magic. They’re tools that demand respect. The best outcomes come from patients who get tested regularly, report side effects early, and don’t skip appointments. The worst outcomes? They come from people who think, “I feel fine, so I don’t need blood work.”
If you’re on a JAK inhibitor, know your numbers. Know your risks. Talk to your doctor about alternatives if you’re over 50 or have heart disease. If you’re considering one, ask: Is this the right time? Am I healthy enough? What happens if I get shingles again?
These drugs changed the game. But they didn’t remove risk-they just moved it from the injection room to the lab report.
Are JAK inhibitors safer than biologics?
It depends. JAK inhibitors are easier to take and work faster, but they carry higher risks for heart problems, cancer, and blood clots, especially in older adults or those with existing risk factors. Biologics have their own risks-like infections and injection reactions-but their long-term safety profile is better studied. For patients under 50 with no heart disease, JAK inhibitors can be a great option. For others, biologics are often the safer choice.
How long do I need to take a JAK inhibitor?
There’s no set time limit. Many people stay on them for years if they’re working and side effects are controlled. But if you develop high blood pressure, high cholesterol, or signs of infection, your doctor may switch you. Some patients stop after 1-2 years because of side effects. Others stay on indefinitely. The key is regular monitoring-never assume it’s safe just because you feel good.
Can I get vaccinated while on a JAK inhibitor?
Yes-but only certain vaccines. Live vaccines (like MMR, chickenpox, nasal flu) are dangerous and should be avoided. Inactivated vaccines (flu shot, pneumonia, COVID-19, shingles) are safe and recommended. The shingles vaccine (Shingrix) is especially important-you should get it before starting the drug if possible. Even if you’re already on a JAK inhibitor, you can still get Shingrix. It’s not live, and it reduces your risk of shingles by about 70%.
What should I do if I develop shingles while on a JAK inhibitor?
Call your doctor immediately. Shingles can become severe in people on immunosuppressants. You’ll likely need high-dose antiviral medication and possibly hospitalization. Your JAK inhibitor will probably be paused until the infection clears. After recovery, your doctor will reassess whether to restart it-many choose to switch to a different drug because the risk of recurrence is high.
Why do JAK inhibitors raise cholesterol?
JAK2 plays a role in how the liver processes fats. When JAK2 is blocked, LDL (bad cholesterol) increases and HDL (good cholesterol) may drop. This is a direct effect of the drug, not lifestyle. About 40-45% of users see their LDL rise by 20-40 mg/dL. It’s not optional to treat-it’s part of the safety protocol. If your LDL goes above 190 mg/dL, your doctor should start you on a statin, even if you’ve never had high cholesterol before.
Are there alternatives to JAK inhibitors if I’m worried about risks?
Yes. Newer biologics like IL-17 or IL-23 inhibitors (secukinumab, risankizumab) are now available for psoriasis and arthritis. They’re injectable, but their safety profile is better for older patients and those with heart disease. For alopecia areata, topical treatments and phototherapy are options. If you’re concerned about long-term risks, talk to your rheumatologist or dermatologist about switching. There’s no shame in choosing safety over convenience.

Jessie Ann Lambrecht
January 7, 2026 AT 22:40JAK inhibitors gave me my life back after 12 years of joint pain - but I didn’t skip a single blood test. Ever. Got my LDL up to 204? Started a statin. Got shingles? Paused the med, got antivirals, and now I’m on a lower dose. These aren’t magic pills - they’re tools. Treat them like a chainsaw, not a butter knife.
Rachel Steward
January 9, 2026 AT 14:28Let’s be real - the FDA black box warning exists for a reason. You’re trading a 2-week symptom relief for a 49% higher cancer risk? That’s not a trade, that’s Russian roulette with a lab report. And don’t even get me started on how clinics skip the chickenpox vaccine check. It’s not negligence - it’s profit-driven negligence.
Sai Ganesh
January 11, 2026 AT 06:08In India, many patients can’t even afford biologics, so JAK inhibitors are a lifeline. But access doesn’t mean understanding. I’ve seen patients stop blood tests because they ‘feel fine.’ We need better education - not just more prescriptions. Safety isn’t optional, even when resources are tight.
Paul Mason
January 12, 2026 AT 18:15My mate took upadacitinib for psoriasis. Skin cleared in 3 weeks. Then his cholesterol went nuts. Doc put him on a statin, told him to quit smoking, and now he’s good. No drama. Just check your numbers, don’t be a hero. Simple as that.
steve rumsford
January 14, 2026 AT 02:53so i took ritlecitinib for my alopecia… hair grew back like a damn miracle. then i got shingles. twice. now im on a biologic and my scalp is still 80% covered. worth it? maybe. did i get lucky? absolutely. dont be me.
Andrew N
January 15, 2026 AT 09:13The 31% increase in cardiovascular events is statistically significant. The ORAL Surveillance study was large and long-term. Ignoring this is dangerous. People think ‘I feel fine’ means ‘I’m safe.’ That’s the exact mindset that kills.
LALITA KUDIYA
January 15, 2026 AT 17:57Poppy Newman
January 17, 2026 AT 14:21Shingrix before starting? YES. 🛡️ I got it 6 weeks before my first pill. No shingles. No stress. My doc was impressed. Why is this not standard everywhere? It’s literally the easiest win.
Adam Gainski
January 18, 2026 AT 23:00For those considering JAK inhibitors: if you’re under 50, no heart disease, no smoking, and you’re willing to do the bloodwork - they’re a game-changer. But if you’re over 50, have high BP, or a family history of clots? Walk away. There are safer options. Your future self will thank you.
Anastasia Novak
January 20, 2026 AT 11:45Ugh. Another ‘trust your doctor’ post. Newsflash: doctors are overworked, underpaid, and pressured by pharma reps. They push JAK inhibitors because they’re easy to prescribe, not because they’re safe. If you’re not doing your own research, you’re just a walking risk factor.
Alex Danner
January 22, 2026 AT 00:09Just got my 6-month labs back. LDL up 35, lymphocytes down 120. Doc paused my abrocitinib, started me on a statin, and we’re switching to risankizumab next month. I’m not mad - I’m grateful. This system works if you show up. Show up. Get tested. Stay alive.