For someone living with Crohn’s disease, every day can feel like walking a tightrope between relief and flare-up. The constant cramps, diarrhea, fatigue - it’s not just inconvenient, it’s exhausting. And when medications like steroids or immunomodulators stop working, the question isn’t just what next? It’s can I even get better? The answer, for many, lies in biologic therapy - a game-changer that targets the root of the inflammation, not just the symptoms.
What Exactly Is Crohn’s Disease?
Crohn’s disease is a chronic inflammatory bowel condition that can attack any part of the digestive tract, from the mouth to the anus. Most often, it hits the end of the small intestine (terminal ileum) and the beginning of the colon. Unlike ulcerative colitis, which only affects the innermost lining of the colon, Crohn’s digs deep - all the way through the intestinal wall. This is called transmural inflammation.It’s not caused by bad food or stress, though those can make symptoms worse. At its core, Crohn’s is an autoimmune problem: the immune system mistakes harmless gut bacteria for invaders and launches a relentless attack. Over 200 genes are linked to increased risk, with NOD2/CARD15 mutations found in up to 40% of people with a family history. Environmental triggers - smoking, antibiotics in childhood, diets high in processed fats - can flip the switch in genetically prone people.
The result? Inflamed, swollen tissue that thickens, cracks, and sometimes forms tunnels called fistulas. About 30-50% of people develop strictures (narrowed sections) within 10 years. Around 25-35% get fistulas. These aren’t just uncomfortable - they can lead to abscesses, infections, and emergency surgery.
Why Biologics Are Different From Other Treatments
Before biologics, treatment was a ladder: start with mild drugs like mesalamine, then add immunomodulators like azathioprine, then steroids for flares. But steroids don’t heal the gut - they just silence the noise temporarily. And they come with serious side effects: bone loss, diabetes, mood swings.Biologics are targeted. They’re made from living cells, not chemicals. Instead of broadly suppressing the immune system, they zero in on specific molecules driving inflammation. Think of them as precision missiles instead of carpet bombing.
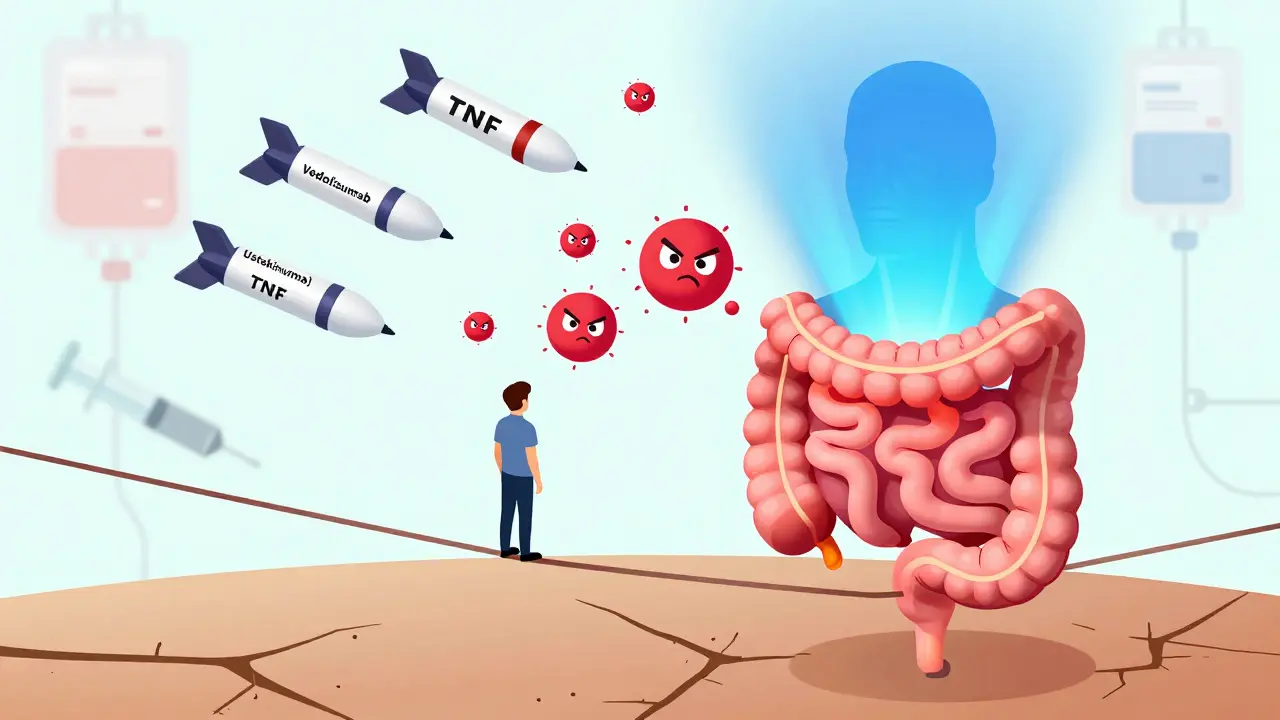
The first big breakthrough came in 1998 with infliximab (Remicade), an anti-TNF-α drug. TNF-alpha is a key inflammatory signal in Crohn’s. Blocking it stopped the firestorm in many patients. Since then, more options have arrived:
- Anti-TNF agents: infliximab, adalimumab (Humira), certolizumab (Cimzia), golimumab
- Vedolizumab (Entyvio): Blocks immune cells from entering the gut - 75% specific to the intestines
- Ustekinumab (Stelara): Targets IL-12 and IL-23, two other major inflammatory messengers
These aren’t just alternatives - they’re more effective. Clinical trials show anti-TNF drugs induce remission in 30-40% of patients, compared to 15-20% with placebo. Vedolizumab and ustekinumab work well too, especially for those who don’t respond to anti-TNFs. And they actually heal the gut lining - something older drugs rarely do.
How Biologics Work: The Science Behind the Shots
Your gut is full of immune cells waiting to fight invaders. In Crohn’s, these cells get confused. They stick around, keep attacking, and recruit more troops. That’s where biologics step in.Anti-TNF drugs like Humira and Remicade latch onto TNF-alpha molecules and neutralize them. No TNF-alpha means less inflammation, less pain, less damage.
Vedolizumab works differently. It blocks a protein called α4β7 integrin - the “passport” immune cells use to enter the gut. By shutting that down, it stops the flood of inflammatory cells without affecting the rest of the body. That’s why it has fewer systemic side effects.
Ustekinumab hits IL-12 and IL-23, two cytokines that drive the T-cell response. These are the same pathways targeted by newer psoriasis drugs. Blocking them reduces inflammation and helps maintain long-term remission.
Each drug has its own rhythm. Infliximab is given as an IV infusion every 8 weeks. Adalimumab is a self-injected shot every two weeks. Vedolizumab is also infused, but takes longer to work - often 10-14 weeks before you feel real change. Anti-TNFs usually respond faster: 2-4 weeks.
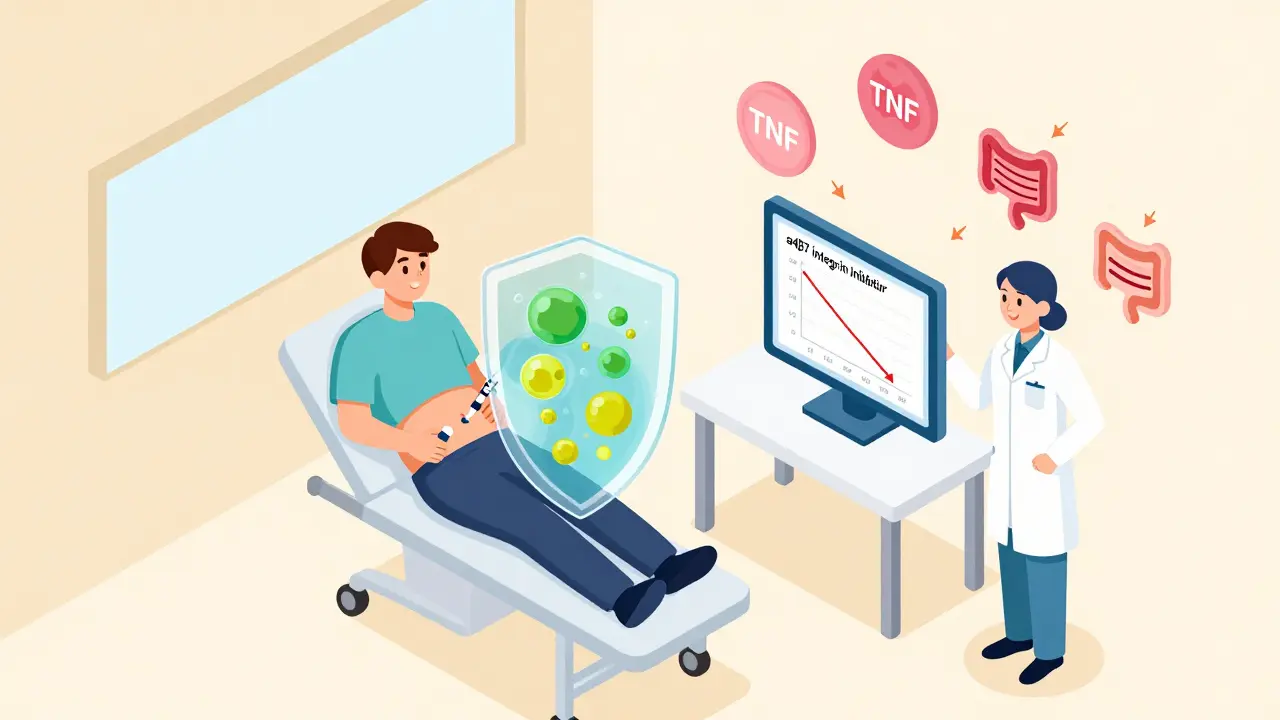
Who Benefits Most From Biologics?
Not everyone with Crohn’s needs a biologic. But if you have:- Deep ulcers seen on colonoscopy
- Strictures or fistulas
- Perianal disease (sores near the anus)
- Failed at least two conventional therapies
- High inflammatory markers (CRP, ESR)
- you’re a strong candidate for early biologic use. Experts now call this the “top-down” approach: start strong to prevent damage before it happens. Studies show patients who begin biologics early cut their risk of surgery by half within five years.
Some people are better suited to certain drugs. If you have joint pain or skin issues (extraintestinal manifestations), vedolizumab might be preferred - it’s less likely to trigger neurological side effects. If you’ve had a bad reaction to anti-TNFs, ustekinumab is a solid second choice.
Cost, Access, and Real-Life Challenges
Let’s be honest: biologics are expensive. Annual costs range from $35,000 to $70,000. In the U.S., 40% of patients delay doses because copays exceed $150 per treatment. Even with insurance, you might face prior authorizations that take weeks. Some people spend hours on the phone with insurers just to get approval.But help exists. Patient assistance programs from drugmakers can cover 30-50% of out-of-pocket costs. Biosimilars - cheaper copies of brand-name biologics like infliximab-dyyb (Inflectra) - are now available and gaining traction. They work the same way, cost 15-30% less, and are approved by the FDA.
Practical hurdles are real too. Infusion centers aren’t always near work. Scheduling around a 9-to-5 job is tough. Self-injections require training - 85% of patients master them after two or three supervised sessions. Some get injection site reactions: redness, swelling, itching. It’s annoying, but usually manageable.
And then there’s the mental toll. About 25-30% of patients report “infusion anxiety” - fear of side effects, panic before appointments, dread of needles. Counseling or cognitive behavioral therapy helps more than people realize.
Side Effects and Safety: What You Need to Know
Biologics are powerful, but not risk-free. The biggest concern? Infections. Because they dampen parts of your immune system, you’re more vulnerable to TB, hepatitis B, and serious bacterial infections. That’s why everyone gets tested for TB and hepatitis before starting.Anti-TNF drugs carry a small risk of reactivating latent TB. A simple blood test (Quantiferon Gold) catches this. Hepatitis screening is standard too.
Some people develop lupus-like symptoms on Humira - joint pain, rashes, fatigue. It’s rare, but serious. If it happens, stopping the drug usually reverses it.
Another issue: immunogenicity. Your body can see the biologic as a foreign invader and build antibodies against it. That’s why some patients lose response over time - the drug stops working. Anti-TNFs have a 30-46% annual loss rate. Vedolizumab? Only 4% develop antibodies. That’s one reason it’s becoming a go-to for long-term use.
Therapeutic drug monitoring helps. Doctors now check blood levels of infliximab and adalimumab to make sure you’re getting enough. Target levels? 3-7 μg/mL for infliximab, 5-12 μg/mL for adalimumab. If you’re below that, your dose can be increased or shortened. This simple step boosts remission rates by 3.5 times.

Real Stories: Hope, Setbacks, and Survival
On Reddit’s Crohn’s community, one user wrote: “Infliximab took me from 15 bowel movements a day to two within three infusions. I went from bedridden to working again.” That’s the dream.Another shared: “Humira gave me lupus-like symptoms after 18 months. I needed six months of steroids to recover.” That’s the nightmare.
Health Union’s 2023 survey of over 1,200 patients showed 78% felt their quality of life improved. Most could keep working. 85% stopped relying on steroids. But 65% struggled with cost. 35% had infusion or injection reactions. 28% worried about long-term infection risk.
These aren’t abstract numbers. They’re real people - moms, dads, students, workers - trying to live full lives while managing a chronic illness.
What’s Next? Emerging Therapies and Hope on the Horizon
The field is moving fast. New drugs are in phase 3 trials. Ozanimod (Zeposia), originally for MS, is showing 37% remission rates in Crohn’s. Mirikizumab, an IL-23p19 inhibitor, achieved 40% endoscopic improvement - meaning visible healing of the gut lining.Biosimilars are expanding access. More insurers are covering them. In the next five years, they could cut costs significantly.
Research is also exploring personalized medicine. Genetic testing might one day tell you which biologic you’re most likely to respond to. Blood biomarkers could predict flares before they happen.
For now, the message is clear: biologics aren’t perfect, but they’re the best tool we have to control Crohn’s disease and prevent irreversible damage. They don’t cure it - but they give you back your life.
Starting Biologic Therapy: What to Expect
If your doctor recommends a biologic, here’s what usually happens:- Testing: TB screen, hepatitis panel, heart evaluation (for anti-TNFs)
- Insurance approval: Can take 2-6 weeks. Your clinic’s IBD nurse will help
- First dose: Infusion (for infliximab or vedolizumab) or injection (for adalimumab)
- Follow-up: Check-ins at 4, 8, and 12 weeks. Blood tests to check drug levels
- Long-term: Maintenance doses every 2-8 weeks, depending on the drug
You’ll get training on self-injection if needed. Most clinics offer nurse-led sessions. Apps like MyIBDCoach help track symptoms, side effects, and appointment reminders.
Don’t be afraid to ask questions. Ask about biosimilars. Ask about cost assistance. Ask what happens if it stops working. Knowledge is power.

Caitlin Foster
December 27, 2025 AT 20:35Okay, but let’s be real-biologics are like renting a Lamborghini to drive to the grocery store. You get there faster, sure, but you’re also terrified you’ll scratch the paint, and your insurance is gonna bill you for a new tire every time you hit a pothole. I’ve been on Humira for 3 years. My gut’s quiet. My bank account? Not so much. Still, I’d rather pay $500 a month than live like a human toilet again.
Todd Scott
December 29, 2025 AT 17:15It’s fascinating how the pharmacodynamics of biologics reflect the evolution of immunology itself-from broad-spectrum immunosuppression to precision targeting of cytokine pathways. The shift from azathioprine to ustekinumab mirrors our growing understanding of Th17 cell dominance in Crohn’s pathogenesis. Vedolizumab’s α4β7 integrin blockade is particularly elegant because it preserves systemic immunity while silencing gut-specific inflammation. That’s not just medicine-it’s immunological choreography. And yes, the cost is obscene, but when you consider the societal burden of lost productivity and repeated hospitalizations, it’s a net savings over time. We’re not just treating symptoms; we’re altering disease trajectories.
Andrew Gurung
December 31, 2025 AT 05:31Wow. So you’re telling me I have to pay $70K a year to not poop blood? 🤡 I’m just here for the drama. My cousin’s on Remicade and now she’s got ‘lupus vibes’ and a Pinterest board called ‘My Autoimmune Aesthetic.’ 😭 She posts selfies with her infusion bag like it’s a new handbag. Meanwhile, I’m over here eating charcoal toast and whispering to my colon like it’s a pet rock. 🤷♂️ #CrohnLife #BiologicOrBust
Paula Alencar
January 1, 2026 AT 14:12It is with profound respect for the scientific rigor behind biologic therapies that I acknowledge their transformative potential in the management of Crohn’s disease. The mechanistic precision of anti-TNF agents, the tissue-specificity of vedolizumab, and the cytokine-targeted action of ustekinumab represent not merely pharmacological advancements, but paradigmatic shifts in patient-centered care. The reduction in surgical interventions, the restoration of mucosal healing, and the reclamation of daily autonomy are not incidental-they are monumental. To those who struggle with cost or access: you are not alone. Advocacy, patient support networks, and biosimilar adoption are critical frontiers in this ongoing battle for dignity and health equity.
Nikki Thames
January 1, 2026 AT 18:30Let me ask you something-why do we treat Crohn’s like it’s a problem to be solved rather than a signal to be understood? You’re throwing expensive biologics at a system that’s screaming for balance. What about gut microbiome restoration? FMT? Low-FODMAP diets? You’re treating the symptom, not the soul. You think a shot is going to fix the trauma of childhood antibiotics or your mother’s gluten-free obsession? This isn’t medicine-it’s denial with an IV drip.
Chris Garcia
January 3, 2026 AT 12:26In Nigeria, we call this kind of treatment ‘rich man’s medicine.’ We have people dying from dysentery because they can’t get clean water, and here we are debating which biologic has the lowest immunogenicity rate. Don’t get me wrong-I’m glad it works. But this isn’t progress if it’s only for the few. Biologics are miracles, yes-but miracles shouldn’t come with a price tag that requires a second mortgage. We need global access, not just glossy clinical trial results. The gut doesn’t care if you’re in Lagos or LA-it just wants to be left alone.
James Bowers
January 3, 2026 AT 14:57There is no justification for the current pricing model of biologics. The development costs are recouped within the first 18 months of market release. The patent extensions and evergreening tactics employed by pharmaceutical companies are ethically indefensible. Furthermore, the lack of mandatory transparency regarding manufacturing costs is a breach of public trust. Biosimilars are not merely alternatives-they are moral imperatives. Until pricing is regulated and disclosure enforced, this is not healthcare-it is corporate exploitation dressed in white coats.
Raushan Richardson
January 4, 2026 AT 02:14I started on Humira last year and honestly? It’s been a game-changer. I used to cancel plans every other weekend. Now I’m hiking with my niece. The injections suck, but I’ve gotten used to them. My nurse taught me how to rotate sites and use the ice trick-total lifesaver. Also, I found a biosimilar through my clinic and saved $1,200 a month. If you’re scared to start, just talk to someone who’s been there. You’re not alone.
Kishor Raibole
January 5, 2026 AT 05:43Biologics are a capitalist fantasy disguised as medical innovation. The entire pharmaceutical-industrial complex thrives on chronic illness. If Crohn’s were cured, the stock prices would collapse. You think they want you healed? No. They want you compliant. They want you on lifelong maintenance. They want you to believe that this is the best you can do. The real cure? A society that doesn’t poison its children with processed food, antibiotics, and stress. But that’s too radical, isn’t it? Better to keep injecting.
John Barron
January 6, 2026 AT 16:01Did you know that anti-TNF drugs can increase the risk of lymphoma by 2-4x? 😱 I read it on a forum. Also, my friend’s mom got a fungal infection from Humira and lost her foot. 🦶 And now she’s on disability. So… yeah. I’m just saying… maybe try yoga? Or maybe your gut is just mad at you. I mean, have you tried journaling? Or fasting? Or drinking bone broth? I read this one guy on Medium who cured his Crohn’s with turmeric and breathwork. 🤔 #BiologicRisks #HolisticHealing
Liz MENDOZA
January 7, 2026 AT 19:25I just want to say thank you for writing this. I’ve been on vedolizumab for 14 months. It took forever to work, but now I’m finally sleeping through the night. I used to feel so alone. Reading this made me feel seen. You’re not just listing drugs-you’re talking about real lives. Keep going. We need more of this.
Anna Weitz
January 9, 2026 AT 04:13Jane Lucas
January 11, 2026 AT 02:48